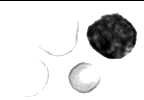
Hypochromia
Definition:
In hypochromia, the erythrocyte central pallor constitutes more than one-third of the cell volume.
Occurrence:
Hypochromia is usually an indication of a decreased hemoglobin concentration (MCHC) as a result of a disorder of hemoglobin synthesis (iron deficiency anemia, thalassemia).
Thursday 29 November 2012
Spherocytes
Spherocytes
Definition:
Spherocytes are ball-shaped erythrocytes. Since they are usually small, they are also called microspherocytes. Unlike microcytes, they have no central pallor and usually have an elevated MCHC. Spherocytes have a reduced life span since they are mechanically-fragile.
Occurrence:
Spherocytes occur naturally in hereditary spherocytosis (a membrane defect) and acquired in hemolytic anemia (loss of cell membrane by immune mechanismes in the RES).
Definition:
Spherocytes are ball-shaped erythrocytes. Since they are usually small, they are also called microspherocytes. Unlike microcytes, they have no central pallor and usually have an elevated MCHC. Spherocytes have a reduced life span since they are mechanically-fragile.
Occurrence:
Spherocytes occur naturally in hereditary spherocytosis (a membrane defect) and acquired in hemolytic anemia (loss of cell membrane by immune mechanismes in the RES).
Oval macrocytes
Oval macrocytes
Definition:
Oval macrocytes are slightly oval, hyperchromic macrocytes.
Occurrence:
Oval macrocytes occur when there is a disturbance in DNA synthesis with vitamin B12 and/or folic acid deficiency (megaloblastic anemia).
Definition:
Oval macrocytes are slightly oval, hyperchromic macrocytes.
Occurrence:
Oval macrocytes occur when there is a disturbance in DNA synthesis with vitamin B12 and/or folic acid deficiency (megaloblastic anemia).
Macrocytosis
Macrocytosis
Definition:
Macrocytosis is the frequent occurrence of macrocytes. These are erythrocytes with a diameter of more than 9 µm and a MCV < fl. Oval macrocytes are typically found in megaloblastic anemia.
Occurrence:
Macrocytosis occurs when abnormal erythropoiesis is present as in alcohol consumption, liver disease, during use of cytotoxic (e.g. azathioprine, hydroxyurea) or virostatic drugs (e.g. zidovudine), in megaloblastic anemia through a vitamin B12 and/or folic acid deficiency as well as in myelodysplastic syndromes.
Definition:
Macrocytosis is the frequent occurrence of macrocytes. These are erythrocytes with a diameter of more than 9 µm and a MCV < fl. Oval macrocytes are typically found in megaloblastic anemia.
Occurrence:
Macrocytosis occurs when abnormal erythropoiesis is present as in alcohol consumption, liver disease, during use of cytotoxic (e.g. azathioprine, hydroxyurea) or virostatic drugs (e.g. zidovudine), in megaloblastic anemia through a vitamin B12 and/or folic acid deficiency as well as in myelodysplastic syndromes.
Microcytosis
Microcytosis
Definition:
Microcytosis is the frequent occurrence of microcytes. These are erythrocytes with a diameter less than 6 µm and a MCV > fl in which the central pallor is still visible.
Occurrence:
Microcytosis occurs in disorders of hemoglobin synthesis such as iron deficiency anemia or thalassemia.
Definition:
Microcytosis is the frequent occurrence of microcytes. These are erythrocytes with a diameter less than 6 µm and a MCV > fl in which the central pallor is still visible.
Occurrence:
Microcytosis occurs in disorders of hemoglobin synthesis such as iron deficiency anemia or thalassemia.
Anisocytosis
Anisocytosis
Definition:
Anisocytosis describes the variety of sizes of erythrocytes (e.g. simultaneous occurrence of microcytes and macrocytes in the peripheral film).
Occurrence:
Typically occurs in recovery from microcytic anemia, i.e. with the release of young erythrocytes which are larger. An excellent example is iron deficiency anemia after iron therapy. This often occurs in other forms of anemia as well.
Definition:
Anisocytosis describes the variety of sizes of erythrocytes (e.g. simultaneous occurrence of microcytes and macrocytes in the peripheral film).
Occurrence:
Typically occurs in recovery from microcytic anemia, i.e. with the release of young erythrocytes which are larger. An excellent example is iron deficiency anemia after iron therapy. This often occurs in other forms of anemia as well.
Parameters of hemolysis
Parameters of hemolysis
In the investigation of hemolysis, certain tests reflect increased cell destruction and others increased activity of erythropoiesis.
In the first group are:
bilirubin - LDH - haptoglobin
The tests of the second group are dicussed in the unit "Red blood cell parameters". They are:
reticulocyte count - red cell production index
The direct and indirect Coombs test are used to demonstrate anti-erythrocytic autoantibodies.
Bilirubin:
Bilirubin is a degradation product of the heme. The breakdown of hemoglobin generates up to 80% of bilirubin and the breakdown of myoglobin generates the remaining 20%. Bilirubin is glucuronated in the liver and immediately excreted in the bile. The glucuronated form is also called conjugated or direct and the non-glucuronated form is also called unconjugated or indirect. In hemolytic disease, unconjugated bilirubin is increased. Unconjugated bilirubin is a sensitive but nonspecific test.
Normal range: Total Bilirubin µmol/L, direct (conjugated) Bilirubin µmol/L.
Lactate dehydrogenase
Lactate dehydrogenase (LDH) is an enzyme that is present in many cells and in erythrocytes too. LDH is increased in hemolysis. Similar to the unconjugated bilirubin, LDH is a sensitive but nonspecific test.
Normal range: IU/L.
Haptoglobin
Haptoglobin binds free hemoglobin., If this occurrs the hemoglobin-haptoglobin complex is immediatly removed by the liver. Haptoglobin is produced by the liver and therefore decreased in disorders of Liver function. Haptoglobin too is a sensitive but nonspecific test.
Normal range: - g/L.
Direct antiglobulin test (DAT or direct Coombs test)
With the DAT, antibodies and complement components fixed in vivo to the surface of red blood cells can be demonstrated. The basic principle of the DAT is the agglutination of patient erythrocytes. This is induced be adding antiglobulin serum (Coombs serum), which can be polyspecific (Anti-IgG and Anti-complement; upper image, gel test) or for further differentiation monospecific (Anti-IgG1-4, -IgA, -IgM, -C3c, -C3d; lower image).
A positve DAT allows only the conclusion that immunoglobulin and/or complement are fixed to the surface of the patient erythrocytes (up to 3-15% of in-patients and 1/1000 to 1/14 000 of healthy blood donors have a positve DAT). Therefore, the interpretation depends also on clinical findings and other lab examinations. A positive DAT is mainly found in autoimmune hemolytic anemia, drug induced immune hemolytic anemia, hemolytic transfusion reactions and beim hemolytic disease of the newborn.
Indirect antiglobulin test (IAT or indirect Coombs test)
With the IAT the presence of free anti-erythrocytic autoantibodies in the serum is detected. In contrast to the DAT, the patient's serum is incubated with normal erythrocytes. Then the indirect Coombs test is performed and interpreted like the direct Coombs test.
A positive IAT indicates, that antibodies against known antigens on the normal erythrocytes are present. Dies test is used for antibody screening or cross-matching before a blood transfusion, for antibody screening in pregnancy, for demonstration of special blood group characteristics or for the screening for free antibodies if the DAT is positive.
In the investigation of hemolysis, certain tests reflect increased cell destruction and others increased activity of erythropoiesis.
In the first group are:
bilirubin - LDH - haptoglobin
The tests of the second group are dicussed in the unit "Red blood cell parameters". They are:
reticulocyte count - red cell production index
The direct and indirect Coombs test are used to demonstrate anti-erythrocytic autoantibodies.
Bilirubin:
Bilirubin is a degradation product of the heme. The breakdown of hemoglobin generates up to 80% of bilirubin and the breakdown of myoglobin generates the remaining 20%. Bilirubin is glucuronated in the liver and immediately excreted in the bile. The glucuronated form is also called conjugated or direct and the non-glucuronated form is also called unconjugated or indirect. In hemolytic disease, unconjugated bilirubin is increased. Unconjugated bilirubin is a sensitive but nonspecific test.
Normal range: Total Bilirubin µmol/L, direct (conjugated) Bilirubin µmol/L.
Lactate dehydrogenase
Lactate dehydrogenase (LDH) is an enzyme that is present in many cells and in erythrocytes too. LDH is increased in hemolysis. Similar to the unconjugated bilirubin, LDH is a sensitive but nonspecific test.
Normal range: IU/L.
Haptoglobin
Haptoglobin binds free hemoglobin., If this occurrs the hemoglobin-haptoglobin complex is immediatly removed by the liver. Haptoglobin is produced by the liver and therefore decreased in disorders of Liver function. Haptoglobin too is a sensitive but nonspecific test.
Normal range: - g/L.
Direct antiglobulin test (DAT or direct Coombs test)
With the DAT, antibodies and complement components fixed in vivo to the surface of red blood cells can be demonstrated. The basic principle of the DAT is the agglutination of patient erythrocytes. This is induced be adding antiglobulin serum (Coombs serum), which can be polyspecific (Anti-IgG and Anti-complement; upper image, gel test) or for further differentiation monospecific (Anti-IgG1-4, -IgA, -IgM, -C3c, -C3d; lower image).
A positve DAT allows only the conclusion that immunoglobulin and/or complement are fixed to the surface of the patient erythrocytes (up to 3-15% of in-patients and 1/1000 to 1/14 000 of healthy blood donors have a positve DAT). Therefore, the interpretation depends also on clinical findings and other lab examinations. A positive DAT is mainly found in autoimmune hemolytic anemia, drug induced immune hemolytic anemia, hemolytic transfusion reactions and beim hemolytic disease of the newborn.
Indirect antiglobulin test (IAT or indirect Coombs test)
With the IAT the presence of free anti-erythrocytic autoantibodies in the serum is detected. In contrast to the DAT, the patient's serum is incubated with normal erythrocytes. Then the indirect Coombs test is performed and interpreted like the direct Coombs test.
A positive IAT indicates, that antibodies against known antigens on the normal erythrocytes are present. Dies test is used for antibody screening or cross-matching before a blood transfusion, for antibody screening in pregnancy, for demonstration of special blood group characteristics or for the screening for free antibodies if the DAT is positive.
Iron metabolism and its parameters
Iron metabolism and its parameters
Iron metabolism:
Iron is present in the body in the ferrous form (Fe++, well-absorbed), as well as in ferric compounds (storage form). Ferric compounds predominate in food. This is why only around 5-10% of oral iron is absorbed. This rate can be increased to a maximum of 30% in iron deficiency. The daily gastrointestinal absorption of iron is normally 1 mg/d. This corresponds rather precisely to the daily loss attained through exfoliation of skin cells, and cells of the gastrointestinal and urogenital tracts. In the case of menstruating women (15-40 mg loss per menstruation) and pregnant women (requirement per pregnancy approximately 500-1000 mg), iron loss is substantially higher. Iron cannot be actively eliminated from the body. For this reason, iron overload can occur under certain circumstances. Iron is stored in the body in the form of ferritin (water-soluble) and hemosiderin (water-insoluble, stainable with Prussian blue).
Serum iron:
In healthy adults, serum iron is subjected to strong variations. On the other hand, it stabilizes in iron deficiency states at low values and in iron overload at high values. Serum iron is low in both iron deficiency and anemia of chronic disease.
Normal range: - µmol/L
Total iron binding capacity (TIBC):
The total iron binding capacity is increased in iron deficiency, but decreased in anemia of chronic disorders.
Normal range: - µmol/L
Transferrin and transferrin saturation:
One transferrin molecule binds two iron molecules. Thus, the total iron binding capacity (µmol/L) is twice the value of transferrin (µmol/L). The transferrin saturation represents the percentage of the total iron binding capacity that is used by iron. A value of less than % may be indicative of an iron deficiency.
Normal range for transferrin saturation: - % $
Serum ferritin:
Ferritin is a water-soluble complex of apoferritin and ferric hydroxide. The serum ferritin level usually correlates with the available storage iron. A lower value normally suggests depletion of storage iron and therefore iron deficiency. However, a normal value does not exclude iron deficiency. An increased value does not always indicate iron overload, since ferritin can be increased as an acute phase protein in several different states such as liver disease, malignancy and chronic inflammatory processes (see also anemia of chronic disease).
Normal range: men - µg/L, women - µg/L
Iron metabolism:
Iron is present in the body in the ferrous form (Fe++, well-absorbed), as well as in ferric compounds (storage form). Ferric compounds predominate in food. This is why only around 5-10% of oral iron is absorbed. This rate can be increased to a maximum of 30% in iron deficiency. The daily gastrointestinal absorption of iron is normally 1 mg/d. This corresponds rather precisely to the daily loss attained through exfoliation of skin cells, and cells of the gastrointestinal and urogenital tracts. In the case of menstruating women (15-40 mg loss per menstruation) and pregnant women (requirement per pregnancy approximately 500-1000 mg), iron loss is substantially higher. Iron cannot be actively eliminated from the body. For this reason, iron overload can occur under certain circumstances. Iron is stored in the body in the form of ferritin (water-soluble) and hemosiderin (water-insoluble, stainable with Prussian blue).
Serum iron:
In healthy adults, serum iron is subjected to strong variations. On the other hand, it stabilizes in iron deficiency states at low values and in iron overload at high values. Serum iron is low in both iron deficiency and anemia of chronic disease.
Normal range: - µmol/L
Total iron binding capacity (TIBC):
The total iron binding capacity is increased in iron deficiency, but decreased in anemia of chronic disorders.
Normal range: - µmol/L
Transferrin and transferrin saturation:
One transferrin molecule binds two iron molecules. Thus, the total iron binding capacity (µmol/L) is twice the value of transferrin (µmol/L). The transferrin saturation represents the percentage of the total iron binding capacity that is used by iron. A value of less than % may be indicative of an iron deficiency.
Normal range for transferrin saturation: - % $
Serum ferritin:
Ferritin is a water-soluble complex of apoferritin and ferric hydroxide. The serum ferritin level usually correlates with the available storage iron. A lower value normally suggests depletion of storage iron and therefore iron deficiency. However, a normal value does not exclude iron deficiency. An increased value does not always indicate iron overload, since ferritin can be increased as an acute phase protein in several different states such as liver disease, malignancy and chronic inflammatory processes (see also anemia of chronic disease).
Normal range: men - µg/L, women - µg/L
Red blood cell parameters
Red blood cell parameters
Hemoglobin - hematocrit - erythrocyte count - erythrocyte indices - reticulocyte count - red cell production index - Erythrocyte sedimentation rate
Hemoglobin:
Hemoglobin concentration is a more precise term than hemoglobin since the amount of hemoglobin per unit volume (L) is measured. The hemoglobin concentration can vary considerably depending on the patient's state of hydration. Hemoglobin is usually measured spectrophotometrically.
Normal range: Men - g/L, women - g/L.
Hematocrit:
Hematocrit corresponds to the percentage of the cells in the blood volume. This value is expressed as a fraction (liter/liter) or in percent. Hematocrit is measured by the direct reading of a blood-filled tube that has previously been centrifuged, or it is calculated from the hemoglobin by an electronic cell counting device.
Normal range: Men - L/L or - %, women - L/L or - %. The general standard value is 0.45 L/L or 45%.
Erythrocyte or red cell count:
The erythrocyte count deals with red cell concentration per liter. The measurement can be done microscopically with a counting chamber or by means of an automated cell counter. In both methods, the leukocytes are counted too. Therefore, if the leukocyte count exceeds 100 x 109/L a correction of the erythrocyte count is necessary.
Normal range: Men - x 1012/L, women - x 1012/L.
Erythrocyte indices:
Erythrocyte indices are derived from the values of hemoglobin (Hb), hematocrit (Hc) and the erythrocyte count (Ec). They reflect erythrocyte volume and hemoglobin concentration in the erythrocyte.
Erythrocyte indices allow anemias to be defined more precisely. Therefore, based on the MCV, anemias can be classified as microcytic (iron deficiency anemia, thalassemia), normocytic (anemia of chronic disorders, anemia of renal disease), and macrocytic (megaloblastic anemia). The MCH classifies hypochromic (iron deficiency anemia, thalassemia) and normochromic (anemia of chronic disorders, anemia of renal disease). Usually, although not always, the terms microcytic/hypochromic, normocytic/normochromic and macrocytic/hyperchromic are paired.
Reticulocyte count
Since reticulocytes are not recognized by Wright staining, they must be made visible by the so-called supravital staining (staining of a non-fixed film). In this staining, the reticulocytes' net-like precipitates are recognizable. The reticulocytes are counted by microscopy in relation to the erythrocytes. The normal value is 0.5 to 2% of the erythrocytes. This value corresponds to the observation that the reticulocytes need around 1-2 days (approximately one hundredth of the erythrocytes' life span) to mature into erythrocytes. In absolute numbers, the normal range is - x 109/L.
Red cell production index
The younger the reticulocytes are when they are released from the bone marrow, the longer the maturation time and the longer the period of time in which they can be detected in the peripheral blood. Since this leads to falsely high values, it is recommended that a correction be done if polychromasia is present which indicates increased erythropoiesis.
Erythrocyte sedimentation rate
The sedimentation rate does not actually belong to the parameters of the red blood picture. However, since results of the sedimentation rate depend on different alterations of the erythrocytes, it will be discussed here. In cases of anemias and macrocytsis it is increased, while in cases of polycythemia, microcytosis and acanthocythosis is is diminished. An elevated fibrinogen (inflammation, infections, neoplasias) also leads to an increase of the erythrocyte sedimentation rate.
The erythrocyte sedimentation rate is an inexpensive test that can be performed quickly. Primarily, it is a non-specific indicator of chronic inflammatory disorders and can indicate the extent of the disorder. Since it increases with a 24-hour delay, a C-reactive protein assay is preferred in many places in acute inflammatory disorders. For diagnosis and follow-up of polymyalgia rheumatica and temporary arthritis, it is vitally important. In oncology, it is considered to be an important indicator of a relapse of Hodgkin disease. An elevated sedimentation rate due to paraproteins is seen in multiple myeloma. A sedimentation rate over 100 mm/h is almost always seen with a fatal underlying disease.
Normal range: Men - mm/1h, women - mm/1h.
Hemoglobin - hematocrit - erythrocyte count - erythrocyte indices - reticulocyte count - red cell production index - Erythrocyte sedimentation rate
Hemoglobin:
Hemoglobin concentration is a more precise term than hemoglobin since the amount of hemoglobin per unit volume (L) is measured. The hemoglobin concentration can vary considerably depending on the patient's state of hydration. Hemoglobin is usually measured spectrophotometrically.
Normal range: Men - g/L, women - g/L.
Hematocrit:
Hematocrit corresponds to the percentage of the cells in the blood volume. This value is expressed as a fraction (liter/liter) or in percent. Hematocrit is measured by the direct reading of a blood-filled tube that has previously been centrifuged, or it is calculated from the hemoglobin by an electronic cell counting device.
Normal range: Men - L/L or - %, women - L/L or - %. The general standard value is 0.45 L/L or 45%.
Erythrocyte or red cell count:
The erythrocyte count deals with red cell concentration per liter. The measurement can be done microscopically with a counting chamber or by means of an automated cell counter. In both methods, the leukocytes are counted too. Therefore, if the leukocyte count exceeds 100 x 109/L a correction of the erythrocyte count is necessary.
Normal range: Men - x 1012/L, women - x 1012/L.
Erythrocyte indices:
Erythrocyte indices are derived from the values of hemoglobin (Hb), hematocrit (Hc) and the erythrocyte count (Ec). They reflect erythrocyte volume and hemoglobin concentration in the erythrocyte.
Erythrocyte indices allow anemias to be defined more precisely. Therefore, based on the MCV, anemias can be classified as microcytic (iron deficiency anemia, thalassemia), normocytic (anemia of chronic disorders, anemia of renal disease), and macrocytic (megaloblastic anemia). The MCH classifies hypochromic (iron deficiency anemia, thalassemia) and normochromic (anemia of chronic disorders, anemia of renal disease). Usually, although not always, the terms microcytic/hypochromic, normocytic/normochromic and macrocytic/hyperchromic are paired.
Reticulocyte count
Since reticulocytes are not recognized by Wright staining, they must be made visible by the so-called supravital staining (staining of a non-fixed film). In this staining, the reticulocytes' net-like precipitates are recognizable. The reticulocytes are counted by microscopy in relation to the erythrocytes. The normal value is 0.5 to 2% of the erythrocytes. This value corresponds to the observation that the reticulocytes need around 1-2 days (approximately one hundredth of the erythrocytes' life span) to mature into erythrocytes. In absolute numbers, the normal range is - x 109/L.
Red cell production index
The younger the reticulocytes are when they are released from the bone marrow, the longer the maturation time and the longer the period of time in which they can be detected in the peripheral blood. Since this leads to falsely high values, it is recommended that a correction be done if polychromasia is present which indicates increased erythropoiesis.
Erythrocyte sedimentation rate
The sedimentation rate does not actually belong to the parameters of the red blood picture. However, since results of the sedimentation rate depend on different alterations of the erythrocytes, it will be discussed here. In cases of anemias and macrocytsis it is increased, while in cases of polycythemia, microcytosis and acanthocythosis is is diminished. An elevated fibrinogen (inflammation, infections, neoplasias) also leads to an increase of the erythrocyte sedimentation rate.
The erythrocyte sedimentation rate is an inexpensive test that can be performed quickly. Primarily, it is a non-specific indicator of chronic inflammatory disorders and can indicate the extent of the disorder. Since it increases with a 24-hour delay, a C-reactive protein assay is preferred in many places in acute inflammatory disorders. For diagnosis and follow-up of polymyalgia rheumatica and temporary arthritis, it is vitally important. In oncology, it is considered to be an important indicator of a relapse of Hodgkin disease. An elevated sedimentation rate due to paraproteins is seen in multiple myeloma. A sedimentation rate over 100 mm/h is almost always seen with a fatal underlying disease.
Normal range: Men - mm/1h, women - mm/1h.
Reticulocytes
Reticulocytes
Definition:
Reticulocytes are young erythrocytes which have just left the bone marrow and have not completely matured.
Appearance:
Although they are a little larger than normal erythrocytes and the central pallor is is just barely formed, reticulocytes cannot be identified by Wright staining. An important feature of their immaturity is the persistence of RNA, that can be verified with supravital staining. The reticular pattern that becomes visible in this staining played a role in the naming of reticulocytes.
Normal range:
Reticulocytes usually constitute 0.5 to 2% of erythrocytes. Their normal range is - x 109/L. A reticulocyte count above the normal range is known as reticulocytosis.
Definition:
Reticulocytes are young erythrocytes which have just left the bone marrow and have not completely matured.
Appearance:
Although they are a little larger than normal erythrocytes and the central pallor is is just barely formed, reticulocytes cannot be identified by Wright staining. An important feature of their immaturity is the persistence of RNA, that can be verified with supravital staining. The reticular pattern that becomes visible in this staining played a role in the naming of reticulocytes.
Normal range:
Reticulocytes usually constitute 0.5 to 2% of erythrocytes. Their normal range is - x 109/L. A reticulocyte count above the normal range is known as reticulocytosis.
Normal erythrocytes
Normal erythrocytes
Appearance:
Erythrocytes comprise quantitatively the largest portion of the peripheral blood cell mass. Their normal range is between - x 1012/L. Erythrocytes are easily recognized. They stain eosinophilic, i.e. they are orange-red, round, have a central pallor and have no nucleus. Erythrocytes normally exhibit high uniformity which is why they are suitable as a standard for other cells. Less than 10% normally show an abnormality in size, shape, or color.
Size:
Erythrocytes measure about 7 µm in diameter. In order to identify size abnormalities, the nucleus of a small lymphocyte can be used as a norm. The shortest diameter of the red cell is approximately 7-8 µm.
Shape:
The erythrocyte form resembles a disc, that is indented in the middle on both sides, or concavo-concave. This creates the central pallor.
Color:
The erythrocyte's color indicates that they are filled with hemoglobin. The central pallor usually constitutes 1/3 of its diameter. If the diameter measures more than one third, then hypochromia is present, i.e. the erythrocytes appear brighter and/or less well-stained. Hyperchromia can also occur. In this case, the erythrocytes appear darker and the central pallor is either absent (spherocyte) or very small. This is only observed in hereditary spherocytosis.
Normochromic
hypochromic
hyperchromic
Inclusions:
Erythrocytes have a uniform cytoplasmic staining. Erythrocytic inclusions are always pathological when present in the peripheral blood.
Function:
Erythrocytes are extraordinary cells in many respects. They transport oxygen and other molecules. The transportation of immune complexes on the cell surface to the spleen and liver, for example, is important. The erythrocyte shape has two decisive advantages. First, an ideal volume-surface ratio results that is important for the exchange of gases. Second, this shape provides for deformability, which is of decisive importance for passage through capillaries. Not having a nucleus also provides a number of advantages. This helps make deformability easier. The heart also benefits, which the following theoretical calculation illustrates. The heart pumps approximately 3 kg of erythrocytes per minute through a person weighing 75 kg. Since a cell nucleus weighing 40 pg is 50% of the weight of an erythrocyte of 100 pg, this creates a savings of one to one and a half fewer tons per day that the heart has to pump.
Appearance:
Erythrocytes comprise quantitatively the largest portion of the peripheral blood cell mass. Their normal range is between - x 1012/L. Erythrocytes are easily recognized. They stain eosinophilic, i.e. they are orange-red, round, have a central pallor and have no nucleus. Erythrocytes normally exhibit high uniformity which is why they are suitable as a standard for other cells. Less than 10% normally show an abnormality in size, shape, or color.
Size:
Erythrocytes measure about 7 µm in diameter. In order to identify size abnormalities, the nucleus of a small lymphocyte can be used as a norm. The shortest diameter of the red cell is approximately 7-8 µm.
Shape:
The erythrocyte form resembles a disc, that is indented in the middle on both sides, or concavo-concave. This creates the central pallor.
Color:
The erythrocyte's color indicates that they are filled with hemoglobin. The central pallor usually constitutes 1/3 of its diameter. If the diameter measures more than one third, then hypochromia is present, i.e. the erythrocytes appear brighter and/or less well-stained. Hyperchromia can also occur. In this case, the erythrocytes appear darker and the central pallor is either absent (spherocyte) or very small. This is only observed in hereditary spherocytosis.
Normochromic
hypochromic
hyperchromic
Inclusions:
Erythrocytes have a uniform cytoplasmic staining. Erythrocytic inclusions are always pathological when present in the peripheral blood.
Function:
Erythrocytes are extraordinary cells in many respects. They transport oxygen and other molecules. The transportation of immune complexes on the cell surface to the spleen and liver, for example, is important. The erythrocyte shape has two decisive advantages. First, an ideal volume-surface ratio results that is important for the exchange of gases. Second, this shape provides for deformability, which is of decisive importance for passage through capillaries. Not having a nucleus also provides a number of advantages. This helps make deformability easier. The heart also benefits, which the following theoretical calculation illustrates. The heart pumps approximately 3 kg of erythrocytes per minute through a person weighing 75 kg. Since a cell nucleus weighing 40 pg is 50% of the weight of an erythrocyte of 100 pg, this creates a savings of one to one and a half fewer tons per day that the heart has to pump.
Toxic abnormalities of neutrophils and monocytes
Toxic abnormalities of neutrophils and monocytes
Definition:
Intense stimulation of myelopoiesis causes toxic abnormalities in neutrophils to occur. These consist of coarser, reddish to blue-violet toxic granulations (corresponding to the primary granules of myeloid preliminary stages), basophilic inclusions also called Döhle bodies (corresponding to RNA remnants), and in cytoplasmic vacuoles. A shift to the left is also normally present at the same time. Myeloid precursors are often found in blood film which also show toxic abnormalities.
In monocytes, distinct vacuole formation is usually observed.
Occurrence:
Toxic abnormalities in neutrophils and monocytes occur in infections (usually bacterial), in the recovery from myelosuppressive chemotherapy and following growth factor therapy such as granulocyte-colony stimulating factor (G-CSF). Pseudo-Döhle bodies are typically seen in the May-Hegglin anomaly.
Definition:
Intense stimulation of myelopoiesis causes toxic abnormalities in neutrophils to occur. These consist of coarser, reddish to blue-violet toxic granulations (corresponding to the primary granules of myeloid preliminary stages), basophilic inclusions also called Döhle bodies (corresponding to RNA remnants), and in cytoplasmic vacuoles. A shift to the left is also normally present at the same time. Myeloid precursors are often found in blood film which also show toxic abnormalities.
In monocytes, distinct vacuole formation is usually observed.
Occurrence:
Toxic abnormalities in neutrophils and monocytes occur in infections (usually bacterial), in the recovery from myelosuppressive chemotherapy and following growth factor therapy such as granulocyte-colony stimulating factor (G-CSF). Pseudo-Döhle bodies are typically seen in the May-Hegglin anomaly.
Megakaryocyte remnants
Megakaryocyte remnants
Appearance:
Megakaryocyte remnants consist mainly of bare nuclei with coarse chromatin and small, pale residual cytoplasm (platelets). In the peripheral blood, their count is indicated in reference to 100 leukocytes.
Occurrence:
Myeloproliferative and myelodysplastic syndromes.
Appearance:
Megakaryocyte remnants consist mainly of bare nuclei with coarse chromatin and small, pale residual cytoplasm (platelets). In the peripheral blood, their count is indicated in reference to 100 leukocytes.
Occurrence:
Myeloproliferative and myelodysplastic syndromes.
Normoblasts
Normoblasts
Appearance:
Normoblasts are the nucleated precursors of erythrocytes. The cytoplasm changes with the increasing maturity from basophilic, to polychromatophilic to orthochromatic. The nucleus is coarse to pycnotic, compact and partially eccentric.
Counting:
In peripheral blood, the normoblast count is calculated relative to 100 leukocytes. If more than 3-5 normoblasts per 100 leukocytes are found, the white blood count must be corrected (correction for normoblasts).
Correction for normoblasts:
Calculation:
Corrected leukocyte count
=
Automatically-counted leukocytes
100 + microscopically-counted normoblasts
x 100
Example:
Corrected leukocyte count at 15 normoblastes/100 leukocytes
=
47.7 x 109/L
100 + 15
x 100 = 41.5 x 109/L
Occurrence:
The occurrence of normoblasts in the peripheral blood is always pathological. They can be observed in accelerated erythropoiesis (e.g. in severe hemolysis), in bone marrow metastatic disease, or in extramedullary blood formation.
Appearance:
Normoblasts are the nucleated precursors of erythrocytes. The cytoplasm changes with the increasing maturity from basophilic, to polychromatophilic to orthochromatic. The nucleus is coarse to pycnotic, compact and partially eccentric.
Counting:
In peripheral blood, the normoblast count is calculated relative to 100 leukocytes. If more than 3-5 normoblasts per 100 leukocytes are found, the white blood count must be corrected (correction for normoblasts).
Correction for normoblasts:
Calculation:
Corrected leukocyte count
=
Automatically-counted leukocytes
100 + microscopically-counted normoblasts
x 100
Example:
Corrected leukocyte count at 15 normoblastes/100 leukocytes
=
47.7 x 109/L
100 + 15
x 100 = 41.5 x 109/L
Occurrence:
The occurrence of normoblasts in the peripheral blood is always pathological. They can be observed in accelerated erythropoiesis (e.g. in severe hemolysis), in bone marrow metastatic disease, or in extramedullary blood formation.
Leukemic blasts
Leukemic blasts
Appearance:
Leukemic blasts can vary in size and appearance depending on the type of leukemia, however, they are usually very similar to one another. This and the decrease of the remaining cells in the peripheral blood lead to the characteristically monotonous picture of the acute leukemias. Except for the leukemic blasts, there are few mature granulocytes present. In other words, no intermediate satges of maturation can be observered as in chronic myelocytic leukemia. The nuclear cytoplasm is typically minimal. The nucleus exhibits a finely distributed chromatin with 1 to several, distinct nucleolei in most cases. The presence of granules is typical for acute myelocytic leukemia, while the presence of Auer rods is diagnostic. In cases where a specific type of leukemia (acute lymphocytic or acute myelocytic) cannot be classified morphologically, special cytochemical stains (e.g. peroxidase stain) and the determination of lineage-specific antigens on the cell surface of the blasts by means of flow cytometry can help.
Occurrence:
Acute myelocytic or lymphocytic leukemia.
Appearance:
Leukemic blasts can vary in size and appearance depending on the type of leukemia, however, they are usually very similar to one another. This and the decrease of the remaining cells in the peripheral blood lead to the characteristically monotonous picture of the acute leukemias. Except for the leukemic blasts, there are few mature granulocytes present. In other words, no intermediate satges of maturation can be observered as in chronic myelocytic leukemia. The nuclear cytoplasm is typically minimal. The nucleus exhibits a finely distributed chromatin with 1 to several, distinct nucleolei in most cases. The presence of granules is typical for acute myelocytic leukemia, while the presence of Auer rods is diagnostic. In cases where a specific type of leukemia (acute lymphocytic or acute myelocytic) cannot be classified morphologically, special cytochemical stains (e.g. peroxidase stain) and the determination of lineage-specific antigens on the cell surface of the blasts by means of flow cytometry can help.
Occurrence:
Acute myelocytic or lymphocytic leukemia.
Myeloblasts
Myeloblasts
Appearance:
Myeloblasts are the first developmental stage of granulopoiesis. Myeloblasts are 15 - 20 µm in diameter; have a basophilic cytoplasm without granules; and a nucleus with fine, slightly structured chromatin and several noticeable nucleoli.
Occurrence:
- Severe bacterial infections with so-called leukemoid reaction.
- Chronic myelocytic leukemia and myelofibrosis with extramedullary hematopoieis.
- Acute myelocytic leukemias.
Appearance:
Myeloblasts are the first developmental stage of granulopoiesis. Myeloblasts are 15 - 20 µm in diameter; have a basophilic cytoplasm without granules; and a nucleus with fine, slightly structured chromatin and several noticeable nucleoli.
Occurrence:
- Severe bacterial infections with so-called leukemoid reaction.
- Chronic myelocytic leukemia and myelofibrosis with extramedullary hematopoieis.
- Acute myelocytic leukemias.
Promyelocytes
Promyelocytes
Appearance:
Promyelocytes represent the second developmental stage of granulopoiesis between myeloblasts and myelocytes. Cells vary in size from 15 - 25 µm, have a distinct basophilic cytoplasm with coarse, bright red primary (azurophilic) granules, and often have a perinuclear halo. Nuclei range in shape from round to oval and are usually eccentric, with fine chromatin and several nucleoli.
Occurrence:
- Severe bacterial infections with leukemoid reaction.
- Chronic myelocytic leukemia and myelofibrosis with extramedullary hematopoiesis.
- Acute myelocytic leukemias.
- Acute promyelocytic leukemia.
Appearance:
Promyelocytes represent the second developmental stage of granulopoiesis between myeloblasts and myelocytes. Cells vary in size from 15 - 25 µm, have a distinct basophilic cytoplasm with coarse, bright red primary (azurophilic) granules, and often have a perinuclear halo. Nuclei range in shape from round to oval and are usually eccentric, with fine chromatin and several nucleoli.
Occurrence:
- Severe bacterial infections with leukemoid reaction.
- Chronic myelocytic leukemia and myelofibrosis with extramedullary hematopoiesis.
- Acute myelocytic leukemias.
- Acute promyelocytic leukemia.
Myelocytes
Myelocytes
Appearance:
Myelocytes represent the developmental stage of myelopoiesis between promyelocytes and metamyelocytes. Myelocytes are small with a diameter of 12-18 µm and have few primary granulues if any. The nuclear chromatin is coarser and contains at most one nucleolus. They are best distinguished by the form of the nucleus which is only slightly indented. The spectrum of myelocytes is large and includes immature forms with basophilic cytoplasm to mature forms with light pink cytoplasm. Secondary granules, respectively neutrophilic, eosinophilic or basophilic granules appear in myelocytes.
Please note! The preliminary stages with eosinophilic and basophilic granules are classified as eosinophilic and/or basophilic granulocytes.
Occurrence in the peripheral blood:
- In all myeloproliferative syndromes, particularly in chronic myelocytic leukemia and myelofibrosis.
- In severe infections (leukemoid reaction)..
- In abnormalities of the bone marrow blood barrier.
Appearance:
Myelocytes represent the developmental stage of myelopoiesis between promyelocytes and metamyelocytes. Myelocytes are small with a diameter of 12-18 µm and have few primary granulues if any. The nuclear chromatin is coarser and contains at most one nucleolus. They are best distinguished by the form of the nucleus which is only slightly indented. The spectrum of myelocytes is large and includes immature forms with basophilic cytoplasm to mature forms with light pink cytoplasm. Secondary granules, respectively neutrophilic, eosinophilic or basophilic granules appear in myelocytes.
Please note! The preliminary stages with eosinophilic and basophilic granules are classified as eosinophilic and/or basophilic granulocytes.
Occurrence in the peripheral blood:
- In all myeloproliferative syndromes, particularly in chronic myelocytic leukemia and myelofibrosis.
- In severe infections (leukemoid reaction)..
- In abnormalities of the bone marrow blood barrier.
Metamyelocytes
Metamyelocytes
Appearance:
Metamyelocytes are the stage of myelopoiesis before band granulocytes. A metamyelocyte has a kidney-shaped nucleus, the indentation of which does not go past one third of the transverse diameter of the nucleus. The cytoplasm resembles that of mature granulocytes. Metamyelocytes form the postmitotic pool; this means that they are no longer able to divide.
Occurrence:
- In all myeloproliferative syndromes, in particular in chronic myelocytic leukemia and myelofibrosis.
- In severe infections with a shift to the left (leukemoid reaction).
- In abnormalities of the bone marrow blood barrier.
Appearance:
Metamyelocytes are the stage of myelopoiesis before band granulocytes. A metamyelocyte has a kidney-shaped nucleus, the indentation of which does not go past one third of the transverse diameter of the nucleus. The cytoplasm resembles that of mature granulocytes. Metamyelocytes form the postmitotic pool; this means that they are no longer able to divide.
Occurrence:
- In all myeloproliferative syndromes, in particular in chronic myelocytic leukemia and myelofibrosis.
- In severe infections with a shift to the left (leukemoid reaction).
- In abnormalities of the bone marrow blood barrier.
Smudge cells
Smudge cells
Appearance:
Smudge cells are formed when lymphocytes rupture during the preparation of a blood film. These damaged cells are not usually counted. Since damaged lymphocytes often occur in chronic lymphocytic leukemia, they are classified separately in the differential blood count.
Occurrence:
Smudge cells are seen in chronic lymphocytic leukemia, in reactive lymphocytosis (e.g. CMV infection), and other lymphoproliferative processes.
Appearance:
Smudge cells are formed when lymphocytes rupture during the preparation of a blood film. These damaged cells are not usually counted. Since damaged lymphocytes often occur in chronic lymphocytic leukemia, they are classified separately in the differential blood count.
Occurrence:
Smudge cells are seen in chronic lymphocytic leukemia, in reactive lymphocytosis (e.g. CMV infection), and other lymphoproliferative processes.
Atypical lymphocytes
Atypical lymphocytes
Synonym:
Stimulated lymphocytes.
Appearance:
Atypical lymphocytes can vary widely in size, form and shape. They are larger than normal lymphocytes and have a large volume of light to dark basophilic cytoplasm that has a more intense color along the cell membrane. Vacuoles and azure granules may also be present. The nucleus is often kidney-shaped. The chromatin is finer than in small lymphocytes and often has one or more nucleoli. Atypical lymphocytes can not always be clearly identified and are often confused with leukemic blasts by those who are inexperienced.
Occurrence/Presence:
- Viral infections such as infectious mononucleosis, cytomegalovirus and primary HIV infection
- Toxoplasmosis
Synonym:
Stimulated lymphocytes.
Appearance:
Atypical lymphocytes can vary widely in size, form and shape. They are larger than normal lymphocytes and have a large volume of light to dark basophilic cytoplasm that has a more intense color along the cell membrane. Vacuoles and azure granules may also be present. The nucleus is often kidney-shaped. The chromatin is finer than in small lymphocytes and often has one or more nucleoli. Atypical lymphocytes can not always be clearly identified and are often confused with leukemic blasts by those who are inexperienced.
Occurrence/Presence:
- Viral infections such as infectious mononucleosis, cytomegalovirus and primary HIV infection
- Toxoplasmosis
Plasma cells
Plasma cells
Appearance:
Plasma cells are rarely found in peripheral blood. With a diameter of 15-20 µm, they are larger than lymphocytes. Eccentric nuclei and perinuclear halo are typical features of plasma cells. Unlike the histological preparation, the so-called spoke structure of the nucleus cannot be seen in the aspirate.
Normal range:
The normal range of plasma cells is to x 109/L. Consequently, they are rarely found in the blood film. An increase in the number of plasma cells is called plasmacytosis.
Function:
Plasma cells produce antibodies for defense against infection.
Clinical Significance:
Plasma calls are rarely found in peripheral blood. When they are found, however, it is most often following vaccinations.
Benign plasmacytosis occurs in cases of viral infections such as the German measles. In patients with advanced plasmacytoma, a release of plasma cells into the peripheral blood occurs from time to time (plasma cell leukemia).
Appearance:
Plasma cells are rarely found in peripheral blood. With a diameter of 15-20 µm, they are larger than lymphocytes. Eccentric nuclei and perinuclear halo are typical features of plasma cells. Unlike the histological preparation, the so-called spoke structure of the nucleus cannot be seen in the aspirate.
Normal range:
The normal range of plasma cells is to x 109/L. Consequently, they are rarely found in the blood film. An increase in the number of plasma cells is called plasmacytosis.
Function:
Plasma cells produce antibodies for defense against infection.
Clinical Significance:
Plasma calls are rarely found in peripheral blood. When they are found, however, it is most often following vaccinations.
Benign plasmacytosis occurs in cases of viral infections such as the German measles. In patients with advanced plasmacytoma, a release of plasma cells into the peripheral blood occurs from time to time (plasma cell leukemia).
Lymphocytes
Lymphocytes
Appearance:
Lymphocytes have a round to oval nucleus and are approximately the size of erythrocytes. Cytoplasm is grey-blue. The volume of cytoplasm varies widely. As a result, cell size varies between 9 and 20 µm. Large and small lymphocytes are distinguished by the volume of cytoplasm. The difference in size is an expression of different states of activation.
Normal range:
The normal lymphocyte count lies between and x 109/L. Therefore, they are the second most frequent leukocyte in the blood film after neutrophilic granulocytes. If the lymphocyte count is low, lymphopenia is present. If it is increased, then lymphocytosis is present. Morphologically, B and T lymphocytes cannot be distinguished from one another.
Function:
Lymphocytes participate in cellular and humoral defense against infection.
Special form: Large granular lymphocytes
A special lymphocyte form is the large granular lymphocytes, or LGL for short. These cells have a large volume of cytoplasm with clear azure granules. They constitute 5-10% of all lymphocytes. They correspond to either a subpopulation of T-cells or natural killer cells (NK cells).
Clincal Significance:
Lymphocytosis may occur primarily in the case of lymphoproliferative syndromes or secondarily, i.e. reactively, in the case of infections (e.g. pertussis, mononucleosis, and other viral infections), stress (e.g. myocardial infarct) and chronic inflammatory diseases.
Lymphopenia can occur in cases of infections (e.g. HIV, tuberculosis), radiotherapy and systemic diseases as well as during immune suppressive therapy.
Appearance:
Lymphocytes have a round to oval nucleus and are approximately the size of erythrocytes. Cytoplasm is grey-blue. The volume of cytoplasm varies widely. As a result, cell size varies between 9 and 20 µm. Large and small lymphocytes are distinguished by the volume of cytoplasm. The difference in size is an expression of different states of activation.
Normal range:
The normal lymphocyte count lies between and x 109/L. Therefore, they are the second most frequent leukocyte in the blood film after neutrophilic granulocytes. If the lymphocyte count is low, lymphopenia is present. If it is increased, then lymphocytosis is present. Morphologically, B and T lymphocytes cannot be distinguished from one another.
Function:
Lymphocytes participate in cellular and humoral defense against infection.
Special form: Large granular lymphocytes
A special lymphocyte form is the large granular lymphocytes, or LGL for short. These cells have a large volume of cytoplasm with clear azure granules. They constitute 5-10% of all lymphocytes. They correspond to either a subpopulation of T-cells or natural killer cells (NK cells).
Clincal Significance:
Lymphocytosis may occur primarily in the case of lymphoproliferative syndromes or secondarily, i.e. reactively, in the case of infections (e.g. pertussis, mononucleosis, and other viral infections), stress (e.g. myocardial infarct) and chronic inflammatory diseases.
Lymphopenia can occur in cases of infections (e.g. HIV, tuberculosis), radiotherapy and systemic diseases as well as during immune suppressive therapy.
Monocytes
Monocytes
Appearance:
With a diameter of 15 to 20 µm, monocytes are the largest cells in the peripheral blood. Their form is diverse. Monocytes may have pseudo-podi formation on their outer membrane. The cytoplasm is bluish gray. Fine azure granules and vacuoles are often present as well. The nucleus can be bean-shaped or lobulated. Its chromatin is medium fine.
Normal range:
The normal values range from to x 109/L. An increase of monocytes is called monocytosis; a dercrease is called monocytopenia.
Function:
Monocytes may have a distinct migration ability. When they have emigrated to the tissue, they are referred to as macrophages. Monocytes take on an important role in acute and chronic infections. They actively phagocytize and are important components of cell-mediated immunity.
Clinical Significance:
Monocytosis is associated with various chronic infections (e.g. tuberculosis, typhoid fever), chronic inflammatory and malignant diseases (e.g. M. Hodgkins). Monocytosis can likewise be found in acute myelomonocytic leukemia (M4 after FAB) as well as in chronic myelomonocytic leukemia.
Monocytopenia is present in bone marrow aplasia, hairy cell leukemia and in steroid therapy..
Table of Contents
Appearance:
With a diameter of 15 to 20 µm, monocytes are the largest cells in the peripheral blood. Their form is diverse. Monocytes may have pseudo-podi formation on their outer membrane. The cytoplasm is bluish gray. Fine azure granules and vacuoles are often present as well. The nucleus can be bean-shaped or lobulated. Its chromatin is medium fine.
Normal range:
The normal values range from to x 109/L. An increase of monocytes is called monocytosis; a dercrease is called monocytopenia.
Function:
Monocytes may have a distinct migration ability. When they have emigrated to the tissue, they are referred to as macrophages. Monocytes take on an important role in acute and chronic infections. They actively phagocytize and are important components of cell-mediated immunity.
Clinical Significance:
Monocytosis is associated with various chronic infections (e.g. tuberculosis, typhoid fever), chronic inflammatory and malignant diseases (e.g. M. Hodgkins). Monocytosis can likewise be found in acute myelomonocytic leukemia (M4 after FAB) as well as in chronic myelomonocytic leukemia.
Monocytopenia is present in bone marrow aplasia, hairy cell leukemia and in steroid therapy..
Table of Contents
Basophils
Basophils
Appearance:
The basophilic granulocyte at 10 to 14 µm is smaller than the other granulocytes. The coarse, dark violet granules are tightly packed and extensively overlay the nucleus and cytoplasm.
Normal range:
The normal range for basophilis is to x 109/L. Consequently, they are very rarely found in the blood film. An increase of the basophil count is termed basophilia.
Function:
Basophils have an important role to play in hypersensivity reactions. They are also able to leave the bloodstream and to emigrate to the surrounding tissue.
Clinical Significance:
Basophilia is often present in cases of chronic myelocytic leukemia or the other myeloproliferative syndromes, particularly polycythemia vera.
Appearance:
The basophilic granulocyte at 10 to 14 µm is smaller than the other granulocytes. The coarse, dark violet granules are tightly packed and extensively overlay the nucleus and cytoplasm.
Normal range:
The normal range for basophilis is to x 109/L. Consequently, they are very rarely found in the blood film. An increase of the basophil count is termed basophilia.
Function:
Basophils have an important role to play in hypersensivity reactions. They are also able to leave the bloodstream and to emigrate to the surrounding tissue.
Clinical Significance:
Basophilia is often present in cases of chronic myelocytic leukemia or the other myeloproliferative syndromes, particularly polycythemia vera.
Eosinophils
Eosinophils
Appearance:
With a diameter of 16 µm, eosinophil granulocytes are round and slightly larger than neutrophil granulocytes. The granules are coarse, bright red to honey-yellow and very densely packed. The nucleus is usually bilobed.
Normal range:
Eosinophils are rarely found in a blood film and have normal values of - x 109/L. An increase of the eosinophil count is called eosinophilia and a decrease eosinopenia.
Function:
The function of the eosinophils is not completely clear. They play an important role in allergic diseases as well as in parasitic infections. Like neutrophils, they are capable of phagocytosis and migration.
Clinical Significance:
Eosinophilia is usually caused by allergic or parasitic diseases. Many medications can cause eosinophilia as well. Neoplastic diseases (e.g. M. Hodgkins) are occasionaly accompied by eosinophilia. Cases in which values of > 1.5 x 109/L persist, and a cause cannot be established, are refered to as idiopathic eosinophilia syndromes.
Eosinopenia is commonly seen in situations of stress and acute infections. Earlier, reoccurance of eosinophils was considered as sign of recovery from an infectious disease.
Appearance:
With a diameter of 16 µm, eosinophil granulocytes are round and slightly larger than neutrophil granulocytes. The granules are coarse, bright red to honey-yellow and very densely packed. The nucleus is usually bilobed.
Normal range:
Eosinophils are rarely found in a blood film and have normal values of - x 109/L. An increase of the eosinophil count is called eosinophilia and a decrease eosinopenia.
Function:
The function of the eosinophils is not completely clear. They play an important role in allergic diseases as well as in parasitic infections. Like neutrophils, they are capable of phagocytosis and migration.
Clinical Significance:
Eosinophilia is usually caused by allergic or parasitic diseases. Many medications can cause eosinophilia as well. Neoplastic diseases (e.g. M. Hodgkins) are occasionaly accompied by eosinophilia. Cases in which values of > 1.5 x 109/L persist, and a cause cannot be established, are refered to as idiopathic eosinophilia syndromes.
Eosinopenia is commonly seen in situations of stress and acute infections. Earlier, reoccurance of eosinophils was considered as sign of recovery from an infectious disease.
Segmented Neutrophils
Segmented Neutrophils
Appearance:
Neutrophilic granulocytes are usually circular and have a light-grey to pink cytoplasm. The diameter is usually around 14 µm. The granules are very fine and range in color from reddish-violet to brown. The nucleus of band neutrophils is bent and oblong partly with constrictions which have not yet resulted in a filament.
Normal range:
The neutrophil count usually is between and x 109/L. The deviation from this normal range downwards is known as neutropenia and upwards as neutrophilia. If the neutrophil count falls to under 0.5 x 109/L, severe neutropenia is present. This condition can be life-threatening since it can lead to severe infections.
Function:
One of the main tasks of neutrophils is defense against bacterial infections, by phagocytizing the pathogens and killing them. Neutrophils can leave the bloodstream and move into the surrounding tissue, to fight infection. Normaly, they remain in the blood stream for about six hours and in the surrounding tissue for 1-2 days. About half of the neutrophils do not circulate in the peripheral blood, but rather adhere to the walls of smaller vessels (marginal pool).
Segmentation:
Neutrophilic granulocytes can be subdivided by means of their nuclear structure into band neutrophils and segmented neutrophils. There are two different definitions which explain this subdivision.
Definition #1: As soon as the nucleus is threadlike and constricted at any given place, it can be called a segmented neutrophil. Before that it is called a band neutrophil (The rule of filament).
Definition #2: As soon as the diameter of the nucleus at any given place is less than 1/3 that of its widest point, it is a segmented neutrophil (The rule of one third).
Subsequently, different normal values result depending on which definition is used.
Band cells are younger than segmented cells. Neutrophils with more than four constrictions are regarded as hypersegmented. The ratio of band neutrophils to segmented neutrophils is normally around 1 to 4 (the rule of filament), respectively 1 to 12-15 (the rule of one third). If the ratio shifts and there are more band neutrophils, it is called shift to the left or immaturity.
Clinical Significance:
Neutrophilia can have various causes. Mobilization of the marginal pool is typical in stressful situations (stress induced leukocytosis). Acute infections and inflammation result in the mobilization of neutrophils from bone marrow. Younger forms are not prevelent, which is referred to as shift to the left. In addition, toxic alteration can arise especially in cases of bacterial infections. Neutrophilia accompanied by the production/discharge of immature preliminary stages is called a leukemoid reaction. This must be distinguished from chronic myelocytic leukemia, where addittional eosinophilia, basophilia and splenomegaly are typically present. By determining the leukocyte alkaline phosphatase (decreased by CML) one can easily distinguish between the two possible conditions. Chronic infections and inflammation processes may lead to chronically elevated neutrophil counts.
Neutropenia can be caused by reduced production of or increased consumption of neutrophils. Causes of selective neutropenia can include medications (e.g.. non steroidal anti-inflammatory drugs), infections (e.g. parvovirus, typhoid fever, malaria) or autoimmune diseases (e.g. systemic lupus erythematosus). Neutropenia accompanied by anaemia and/or thrombocytopenia occurs in cases of acquired bone marrow aplasia, leukemias, megaloblastic anaemia as well as chemotherapy. Splenomegaly (Hypersplenism) can be an additional cause. Neutropenia can be accompanied by an increased susceptibility to infections. This is especially true if the neutrophil count falls to under 0.5 x 109/L (severe neutropenia).
Hyper segmentation of neutrophils is usually a sign of vitamin B12 or folic acid deficiency (megaloblastic anemia).
Appearance:
Neutrophilic granulocytes are usually circular and have a light-grey to pink cytoplasm. The diameter is usually around 14 µm. The granules are very fine and range in color from reddish-violet to brown. The nucleus of band neutrophils is bent and oblong partly with constrictions which have not yet resulted in a filament.
Normal range:
The neutrophil count usually is between and x 109/L. The deviation from this normal range downwards is known as neutropenia and upwards as neutrophilia. If the neutrophil count falls to under 0.5 x 109/L, severe neutropenia is present. This condition can be life-threatening since it can lead to severe infections.
Function:
One of the main tasks of neutrophils is defense against bacterial infections, by phagocytizing the pathogens and killing them. Neutrophils can leave the bloodstream and move into the surrounding tissue, to fight infection. Normaly, they remain in the blood stream for about six hours and in the surrounding tissue for 1-2 days. About half of the neutrophils do not circulate in the peripheral blood, but rather adhere to the walls of smaller vessels (marginal pool).
Segmentation:
Neutrophilic granulocytes can be subdivided by means of their nuclear structure into band neutrophils and segmented neutrophils. There are two different definitions which explain this subdivision.
Definition #1: As soon as the nucleus is threadlike and constricted at any given place, it can be called a segmented neutrophil. Before that it is called a band neutrophil (The rule of filament).
Definition #2: As soon as the diameter of the nucleus at any given place is less than 1/3 that of its widest point, it is a segmented neutrophil (The rule of one third).
Subsequently, different normal values result depending on which definition is used.
Band cells are younger than segmented cells. Neutrophils with more than four constrictions are regarded as hypersegmented. The ratio of band neutrophils to segmented neutrophils is normally around 1 to 4 (the rule of filament), respectively 1 to 12-15 (the rule of one third). If the ratio shifts and there are more band neutrophils, it is called shift to the left or immaturity.
Clinical Significance:
Neutrophilia can have various causes. Mobilization of the marginal pool is typical in stressful situations (stress induced leukocytosis). Acute infections and inflammation result in the mobilization of neutrophils from bone marrow. Younger forms are not prevelent, which is referred to as shift to the left. In addition, toxic alteration can arise especially in cases of bacterial infections. Neutrophilia accompanied by the production/discharge of immature preliminary stages is called a leukemoid reaction. This must be distinguished from chronic myelocytic leukemia, where addittional eosinophilia, basophilia and splenomegaly are typically present. By determining the leukocyte alkaline phosphatase (decreased by CML) one can easily distinguish between the two possible conditions. Chronic infections and inflammation processes may lead to chronically elevated neutrophil counts.
Neutropenia can be caused by reduced production of or increased consumption of neutrophils. Causes of selective neutropenia can include medications (e.g.. non steroidal anti-inflammatory drugs), infections (e.g. parvovirus, typhoid fever, malaria) or autoimmune diseases (e.g. systemic lupus erythematosus). Neutropenia accompanied by anaemia and/or thrombocytopenia occurs in cases of acquired bone marrow aplasia, leukemias, megaloblastic anaemia as well as chemotherapy. Splenomegaly (Hypersplenism) can be an additional cause. Neutropenia can be accompanied by an increased susceptibility to infections. This is especially true if the neutrophil count falls to under 0.5 x 109/L (severe neutropenia).
Hyper segmentation of neutrophils is usually a sign of vitamin B12 or folic acid deficiency (megaloblastic anemia).
Neutrophils
 Band Neutrophils
Band Neutrophils Appearance:
Neutrophilic granulocytes are usually circular and have a light-grey to pink cytoplasm. The diameter is usually around 14 µm. The granules are very fine and range in color from reddish-violet to brown. The nucleus of band neutrophils is bent and oblong partly with constrictions which have not yet resulted in a filament.
Normal range:
The neutrophil count usually is between and x 109/L. The deviation from this normal range downwards is known as neutropenia and upwards as neutrophilia. If the neutrophil count falls to under 0.5 x 109/L, severe neutropenia is present. This condition can be life threatening since it can lead to severe infections.
Function:
One of the main tasks of neutrophils is defense against bacterial infections, by phagocytizing the pathogens and killing them. Neutrophils can leave the bloodstream and move into the surrounding tissue, to fight infection. Normally, they remain in the blood stream for about six hours and in the surrounding tissue for 1-2 days. About half of the neutrophils do not circulate in the peripheral blood, but rather adhere to the walls of smaller vessels (marginal pool).
Segmentation:
Neutrophilic granulocytes can be subdivided by means of their nuclear structure into band neutrophils and segmented neutrophils. There are two different definitions which explain this subdivision.
Definition #1: As soon as the nucleus is threadlike and constricted at any given place, it can be called a segmented neutrophil. Before that it is called a band neutrophil (The rule of filament).
Definition #2: As soon as the diameter of the nucleus at any given place is less than 1/3 that of its widest point, it is a segmented neutrophil (The rule of one third).
Subsequently, different normal values result depending on which definition is used.
Band cells are younger than segmented cells. Neutrophils with more than four constrictions are regarded as hypersegmented. The ratio of band neutrophils to segmented neutrophils is normally around 1 to 4 (the rule of filament), respectively 1 to 12-15 (the rule of one third). If the ratio shifts and there are more band neutrophils, it is called shift to the left or immaturity.
Clinical Significance:
Neutrophilia can have various causes. Mobilization of the marginal pool is typical in stressful situations (stress induced leukocytosis). Acute infections and inflammation result in the mobilization of neutrophils from bone marrow. Younger forms are prevalent, which is referred to as shift to the left. In addition, toxic alteration can arise especially in cases of bacterial infections. Neutrophilia accompanied by the release of immature preliminary stages is called a leukemoid reaction. This must be distinguished from chronic myelocytic leukemia, where addittional eosinophilia, basophilia and splenomegaly are typically present. By determining the leukocyte alkaline phosphatase (decreased by CML) one can easily distinguish between the two possible conditions. Chronic infections and inflammation processes may lead to chronically elevated neutrophil counts.
Neutropenia can be caused by reduced production of or increased consumption of neutrophils. Causes of selective neutropenia can include medications (e.g.. non steroidal anti-inflammatory drugs), infections (e.g. parvovirus, typhoid fever, malaria) or autoimmune diseases (e.g. systemic lupus erythematosus). Neutropenia accompanied by anaemia and/or thrombocytopenia occurs in cases of acquired bone marrow aplasia, leukemias, megaloblastic anaemia as well as chemotherapy. Splenomegaly (Hypersplenism) can be an additional cause. Neutropenia can be accompanied by an increased susceptibility to infections. This is especially true if the neutrophil count falls to under 0.5 x 109/L (severe neutropenia).
Hyper segmentation of neutrophils is usually a sign of vitamin B12 or folic acid deficiency (megaloblastic anemia).
Multiple trauma-2
-
A revised trauma score results from the sum of respiratory rate, systolic blood pressure, and Glasgow coma scale and can be used to decide which patients should be sent to a trauma center (Table 2.2).Table 2.2. Revised trauma score: trauma scoring systems
Revised Trauma Score (RTS) Rate Score A. Respiratory Rate (breaths/min) 10–29 4 >29 3 6–9 2 1–5 1 0 0 B. Systolic Blood Pressure (mm Hg) >89 4 76–89 3 50–75 2 1–49 1 0 0 C. Glasgow Coma Scale (GCS) Conversion 13–15 4 9–12 3 6–8 2 4–5 1 3 0 RTS = 0.9368 GCS + 0.7326 SBP + 0.2908 RR. The RTS correlates well with the probability of survival.
INJURY SEVERITY SCORE (ISS) (Table
2.3)
Table 2.3. Evaluation of multiple trauma
patient injury severity score (ISS)
| |||||
|---|---|---|---|---|---|
|
-
This anatomic scoring system provides an overall score for patients with multiple injuries.
-
It is based on the Abbreviated Injury Scale (AIS), a standardized system of classification for the severity individual injuries from 1 (mild) to 6 (fatal).
-
Each injury is assigned an AIS score and is allocated to one of six body regions (head, face, chest, abdomen, extremities including pelvis, and external structures).
-
The total ISS score is calculated from the sum of the squares of the three worst regional values. It is important to emphasize that only the worst injury in each body region is used.
-
The ISS ranges from 1 to 75, with any region scoring 6 automatically giving a score of 75.
-
The ISS limits the total number of contributing injuries to three only, one each from the three most injured regions, which may result in underscoring the degree of trauma sustained if a patient has more than one significant injury in more than three regions or multiple severe injuries in one region.
-
To address some of these limitations, Osler et al. proposed a modification to the system which they termed the New Injury Severity Score (NISS). This is defined as the sum of squares of the AIS scores of each of a patient’s three most severe injuries regardless of the body region in which they occur. Both systems
P.16
have been shown to be good predictors of outcome in multiple trauma patients.
EXPOSURE
-
It is important to undress the trauma patient completely and to examine the entire body for signs and symptoms of injury.
RADIOGRAPHIC EVALUATION
A radiographic trauma series consists of the following:
-
Lateral cervical spine: must see all seven vertebrae and the top of T1
-
Can perform swimmer’s view or CT scan if needed.
-
In the absence of adequate cervical spine views of all vertebrae, the cervical spine cannot be “cleared†from possible injury, and a rigid cervical collar must be maintained until adequate views or a CT scan can be obtained.
-
Clinical clearance cannot occur if the patient has a depressed level of consciousness for any reason (e.g., ethanol intoxication).
-
-
Anteroposterior (AP) chest
-
AP pelvis
-
Possibly a lateral thoracolumbar spine
-
Possibly a CT of the head, cervical spine (if not cleared by plain radiographs), thorax, abdomen, or pelvis with or without contrast as dictated by the injury pattern
P.17
STABILIZATION
-
The stabilization phase occurs immediately following initial resuscitation and may encompass hours to days, during which medical optimization is sought. It consists of:
-
Restoration of stable hemodynamics.
-
Restoration of adequate oxygenation and organ perfusion.
-
Restoration of adequate kidney function.
-
Treatment of bleeding disorders.
-
-
Risk of deep venous thrombosis is highest in this period and may be as high as 58% in multiply injured patients. Highest-risk injuries include spinal cord injuries, femur fractures, tibia fractures, and pelvic fractures. A high index of suspicion must be followed by duplex ultrasonography.
-
Low-molecular-weight heparin, or low-dose warfarin has been shown to be more effective than sequential compression devices in preventing thromboses, but it is contraindicated in patients at risk for hemorrhage, especially following head trauma. Prophylaxis should be continued until adequate mobilization of the patient out of bed is achieved.
-
Vena caval filters may be placed at time of angiography and are effective in patients with proximal venous thrombosis.
-
Pulmonary injuries (e.g., contusion), sepsis, multiorgan failure (e.g., because of prolonged shock), massive blood replacement, and pelvic or long bone fractures may result in the adult respiratory distress syndrome (ARDS).
DECISION TO OPERATE
-
Most patients are safely stabilized from a cardiopulmonary perspective within 4 to 6 hours of presentation.
-
Early operative intervention is indicated for:
-
Femur or pelvic fractures, which carry high risk of pulmonary complications (e.g., fat embolus syndrome, ARDS).
-
Active or impending compartment syndrome, most commonly associated with tibia or forearm fractures.
-
Open fractures.
-
Vascular disruption.
-
Unstable cervical or thoracolumbar spine injuries.
-
Patients with fractures of the femoral neck, talar neck, or other bones in which fracture has a high risk of osteonecrosis.
-
-
Determination of patient medical stability
-
Adequacy of resuscitation
-
Vital signs of resuscitation are deceptive.
-
Laboratory parameters include base deficit and lactic acidosis.
-
-
No evidence of coagulopathy
-
As long as homeostasis is maintained, no evidence exists that the duration of the operative procedure results in pulmonary or other organ dysfunction or worsens the prognosis of the patient.
-
Must be ready to change plan as patient status dictates.P.18
-
Patients who are hemodynamically stable without immediate indication for surgery should receive medical optimization (i.e., cardiac risk stratification and clearance) before operative intervention.
-
-
Decision making
-
Determined by general surgery, anesthesia, and orthopaedics.
-
Magnitude of the procedure can be tailored to the patient’s condition.
-
Timing and extent of operative intervention based on physiologic criteria.
-
May require damage control surgery as a temporizing and stabilizing measure.
-
-
Incomplete resuscitation
-
Based on physiologic assessment.
-
Intensive care includes monitoring, resuscitation, rewarming, and correction of coagulopathy and base deficit.
-
Once the patient is warm and oxygen delivery is normalized, reconsider further operative procedures.
-
CONCOMITANT INJURIES
Head Injuries
-
The diagnosis and initial management of head injuries take priority in the earliest phase of treatment.
-
Mortality rates in trauma patients are associated with severe head injury more than any other organ system.
-
Neurologic assessment is accomplished by use of the Glasgow Coma Scale (see earlier).
-
Intracranial pressure monitoring may be necessary.
Evaluation
Emergency computed tomography (CT) scan with or without intravenous
contrast is indicated to characterize the injury radiographically after initial
neurologic assessment.
-
Cerebral contusion
-
Diagnosis: history of prolonged unconsciousness with focal neurologic signs
-
Treatment: close observation
P.19
-
-
Epidural hemorrhage (tear of middle meningeal artery)
-
Diagnosis: loss of consciousness with intervening lucid interval, followed by severe loss of consciousness
-
Treatment: surgical decompression
-
-
Subdural hemorrhage (tear of subdural veins)
-
Diagnosis: neurologic signs may be slow to appear. Lucid intervals may be accompanied by progressive depressed level of consciousness.
-
Treatment: surgical decompression
-
-
Subarachnoid hemorrhage (continuous with cerebrospinal fluid)
-
Diagnosis: signs of meningeal irritation
-
Treatment: close observation
-
Thoracic Injuries
-
These may result from blunt (e.g., crush), penetrating (e.g., gunshot), or deceleration (e.g., motor vehicle accident) mechanisms.
-
Injuries may include disruption of great vessels, aortic dissection, sternal fracture, and cardiac or pulmonary contusions, among others.
-
A high index of suspicion for thoracic injuries must accompany scapular fractures.
-
Emergency thoracotomy may be indicated for severe hemodynamic instability.
-
Chest tube placement may be indicated for hemothorax or pneumothorax.
Evaluation
-
AP chest radiograph may reveal mediastinal widening, hemothorax, pneumothorax, or musculoskeletal injuries.
-
CT with intravenous contrast is indicated with suspected thoracic injuries and may demonstrate thoracic vertebral injuries.
Abdominal Injuries
These may accompany blunt or penetrating trauma.
Evaluation
-
CT scan with oral and intravenous contrast may be used to diagnose intraabdominal or intrapelvic injury. Pelvic fractures, lumbosacral fractures, or hip disorders may be observed.
-
Diagnostic peritoneal lavage remains the gold standard for immediate diagnosis of operable intraabdominal injury.
-
Ultrasound has been increasingly utilized to evaluate fluid present in the abdominal and chest cavities.
-
Positive peritoneal lavage
-
Gross blood, bile, or fecal material
-
>100,000 red blood cells/mL
-
>500 white blood cells/mL
-
Genitourinary Injuries
Fifteen percent of abdominal trauma results in genitourinary
injury.
Evaluation
-
If genitourinary injury is suspected (e.g., blood seen at the urethral meatus), a retrograde urethrogram should be performed before indwelling bladder catheter insertion. Urethral injury may necessitate placement of a suprapubic catheter.
-
If hematuria is present, a voiding urethrogram, cystogram, and intravenous pyelogram are indicated
Multiple Trauma-1
Authors: Koval,
Kenneth J.; Zuckerman, Joseph D.
Title: Handbook
of Fractures, 3rd Edition
Multiple Trauma
-
High-velocity trauma is the number 1 cause of death in the 18- to 44-year age group worldwide.
-
Blunt trauma accounts for 80% of mortality in the <34-year age group.
-
In the 1990s in the United States alone, income loss resulting from death and disability secondary to high-velocity trauma totaled 75 billion dollars annually; despite this, trauma research received less than 2% of the total national research budget.
The polytrauma patient is defined as follows:
-
Injury Severity Score >18
-
Hemodynamic instability
-
Coagulopathy
-
Closed head injury
-
Pulmonary injury
-
Abdominal injury
FIELD TRIAGE
Management Priorities
-
Assessment and establishment of airway and ventilation
-
Assessment of circulation and perfusion
-
Hemorrhage control
-
Patient extrication
-
Shock management
-
Fracture stabilization
-
Patient transport
TRAUMA DEATHS
Trauma deaths tend to occur in three phases:
-
Immediate: This is usually the result of severe brain injury or disruption of the heart, aorta, or large vessels. It is amenable to public health measures and education, such as the use of safety helmets and passenger restraints.
-
Early: This occurs minutes to a few hours after injury, usually as a result of intracranial bleeding, hemopneumothorax, splenic rupture, liver laceration, or multiple injuries with significant blood loss. These represent correctable injuries for which immediate, coordinated, definitive care at a level I trauma center can be most beneficial.
-
Late: This occurs days to weeks after injury and is related to sepsis or multiple organ failure.
GOLDEN HOUR
-
Rapid transport of the severely injured patient to a trauma center is essential for appropriate assessment and treatment.
RESUSCITATION
-
Follows ABCDE
-
Airway, breathing, circulation, disability, exposure
AIRWAY CONTROL
-
The upper airway should be inspected to ensure patency.
-
Foreign objects should be removed, and secretions suctioned.
-
A nasal, endotracheal, or nasotracheal airway should be established as needed. A tracheostomy may be necessary.
-
The patient should be managed as if a cervical spine injury is present. However, no patient should die from lack of an airway because of concern over a possible cervical spine injury. Gentle maneuvers, such as axial traction, are usually possible to allow for safe intubation without neurologic compromise.
BREATHING
-
This involves evaluation of ventilation (breathing) and oxygenation.
-
The most common reasons for ineffective ventilation after establishment of an airway include malposition of the endotracheal tube, pneumothorax, and hemothorax.
-
Tension pneumothorax
-
Diagnosis: tracheal deviation, unilateral absent breath sounds, tympany, and distended neck veins
-
Treatment: insertion of a large-bore needle into the second intercostal space at the midclavicular line; then placement of a chest tube
-
-
Open pneumothorax
-
Diagnosis: sucking chest wound
-
Treatment: occlusive dressing not taped on one side to allow air to escape, followed by surgical wound closure and a chest tube
-
-
Flail chest with pulmonary contusion
-
Diagnosis: paradoxical movement of the chest wall with ventilation
-
Treatment: fluid resuscitation (beware of overhydration); intubation; positive end-expiratory pressure may be necessary
-
-
Endotracheal tube malposition
-
Diagnosis: malposition evident on chest radiograph, unilateral breath sounds, asymmetric chest excursion
-
Treatment: adjustment of the endotracheal tube with or without reintubation
-
-
Hemothorax
-
Diagnosis: opacity on chest radiograph, diminished/ absent breath sounds
-
Treatment: chest tube placement
-
-
-
Indications for intubation
-
Control of airway
-
Prevent of aspiration in an unconscious patient
-
Hyperventilation for increased intracranial pressure
-
Obstruction from facial trauma and edema
-
CIRCULATION
-
Hemodynamic stability is defined as normal vital signs that are maintained with only maintenance fluid volumes.
-
In trauma patients, shock is hemorrhagic until proven otherwise.
-
At a minimum, two large-bore intravenous lines should be placed in the antecubital fossae or groin with avoidance of injured extremities. Alternatively, saphenous vein cutdowns may be used in adults, or intraosseous (tibia) infusion for children <6 years of age.
-
Serial monitoring of blood pressure and urine output is necessary, with possible central access for central venous monitoring or Swan-Ganz catheter placement for hemodynamic instability. Serial hematocrit monitoring should be undertaken until hemodynamic stability is documented.
-
Peripheral blood pressure should be assessed.
-
Blood pressure necessary to palpate a peripheral pulse.
Peripheral pulse Blood pressure Radial 80 mm Hg Femoral 70 mm Hg Carotid 60 mm Hg
INITIAL MANAGEMENT OF THE PATIENT IN SHOCK
-
Direct control of obvious bleeding: direct pressure control preferable to tourniquets or blind clamping of vessels.
-
Large-bore venous access, Ringer lactate resuscitation, monitoring of urine output, central venous pressure, and pH.
-
Blood replacement as indicated by serial hematocrit monitoring.
-
Traction with Thomas splints or extremity splints to limit hemorrhage from unstable fractures.
-
Consideration of angiography (with or without embolization) or immediate operative intervention for hemorrhage control.
DIFFERENTIAL DIAGNOSIS OF HYPOTENSION IN TRAUMA
Cardiogenic Shock
-
Cardiac arrhythmias, myocardial damage
-
Pericardial tamponade
-
Diagnosis: distended neck veins, hypotension, muffled heart sounds (Beck triad)
-
Treatment: pericardiocentesis through subxiphoid approach
-
Neurogenic Shock
-
This occurs in patients with a thoracic level spinal cord injury in which sympathetic disruption results in an inability to maintain vascular tone.
-
Diagnosis: hypotension without tachycardia or vasoconstriction. Consider in a head-injured or spinal cord-injured patient who does not respond to fluid resuscitation.
-
Treatment: volume restoration followed by vasoactive drugs (beware of fluid overload).
Septic Shock
-
Consider in patients with gas gangrene, missed open injuries, and contaminated wounds closed primarily.
-
Diagnosis: hypotension accompanied by fever, tachycardia, cool skin, and multiorgan failure. This occurs in the early to late phases, but not in the acute presentation.
-
Treatment: fluid balance, vasoactive drugs, antibiotics.
Hemorrhagic Shock
-
More than 90% of patients are in shock acutely after trauma.
-
Consider in patients with large open wounds, active bleeding, pelvic and/or femoral fractures, and abdominal or thoracic trauma.
-
Diagnosis: hypotension, tachycardia. In the absence of open hemorrhage, bleeding into voluminous spaces (chest, abdomen, pelvis, thigh) must be ruled out. This may require diagnostic peritoneal lavage, angiography, CT, MRI, or other techniques as dictated by the patient presentation.
-
Treatment: aggressive fluid resuscitation, blood replacement, angiographic embolization, operative intervention, fracture stabilization, and other techniques as dictated by the source of hemorrhage.
CLASSIFICATION OF HEMORRHAGE
| Class I: | <15% loss of circulating blood volume |
| Diagnosis: no change in blood pressure, pulse, or capillary refill | |
| Treatment: crystalloid | |
| Class II: | 15% to 30% loss of circulating blood volume |
| Diagnosis: tachycardia with normal blood pressure | |
| Treatment: crystalloid | |
| Class III: | 30% to 40% loss of circulating blood volume |
| Diagnosis: tachycardia, tachypnea, and hypotension | |
| Treatment: rapid crystalloid replacement, then blood | |
| Class IV: | >40% loss of circulating blood volume |
| Diagnosis: marked tachycardia and hypotension | |
| Treatment: immediate blood replacement |
BLOOD REPLACEMENT
-
Fully cross-matched blood is preferable; it requires approximately 1 hour for laboratory cross-match and unit preparation.
-
Saline cross-matched blood may be ready in 10 minutes; it may have minor antibodies.
-
Type O negative blood is used for life-threatening exsanguination.
-
Warming the blood will help to prevent hypothermia.
-
Monitor coagulation factors, platelets, and calcium levels.
PNEUMATIC ANTISHOCK GARMENT (PASG) OR MILITARY ANTISHOCK TROUSERS
(MAST)
-
Used to control hemorrhage associated with pelvic fractures.
-
May support systolic blood pressure by increasing peripheral vascular resistance.P.14
-
May support central venous pressure by diminution of lower extremity blood pooling.
-
Advantages: simple, rapid, reversible, immediate fracture stabilization.
-
Disadvantages: limited access to the abdomen, pelvis, and lower extremities, exacerbation of congestive heart failure, decreased vital capacity, potential for compartment syndrome.
-
Are contraindicated in patients with severe chest trauma.
INDICATIONS FOR IMMEDIATE SURGERY
Hemorrhage secondary to:
-
Liver, splenic, renal parenchymal injury: laparotomy
-
Aortic, caval, or pulmonary vessel tears: thoracotomy
-
Depressed skull fracture or acute intracranial hemorrhage: craniotomy
DISABILITY (NEUROLOGIC ASSESSMENT)
-
Initial survey consists of an assessment of the patient’s level of consciousness, pupillary response, sensation and motor response in all extremities, rectal tone and sensation.
-
The Glasgow coma scale (Table 2.1) assesses level of consciousness, severity of brain function, brain damage, and potential patient recovery by measuring three behavioral responses: eye opening, best verbal response, and best motor response.Table 2.1. Glasgow coma scale
Glasgow Coma Scale Score A Eye Opening (E) 1. Spontaneous 2. To speech 4 3. To pain 3 4. None 2 B. Best Motor Response (M) 1 1. Obeys commands 2. Localizes to stimulus 6 3. Withdraws to stimulus 5 4. Flexor posturing 4 5. Extensor posturing 3 6. None 2 C. Verbal Response (V) 1 D. Oriented 5 E. Confused conversation 4 F. Inappropriate words 3 G. Incomprehensible phonation 2 H. None 1 GCS = E+M+V (range, 3–15).
Note: Patients with a Glasgow coma scale of <13, a systolic blood pressure of <90, or a respiratory rate of >29 or <10/min should be sent to a trauma center. These injuries cannot be adequately evaluated by physical examination.
Subscribe to:
Posts (Atom)